Key relevant characteristics of Vγ9Vδ2 T-cells include:
- Vg9Vd2 T-cells exist as tumor-infiltrating lymphocytes (TILs) in many different cancer indications
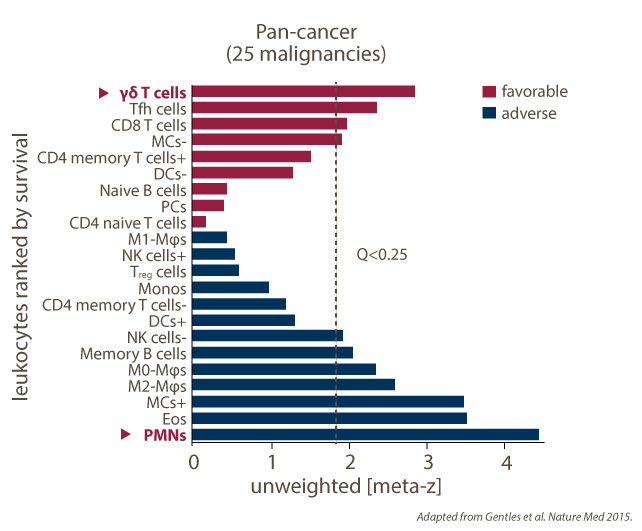
- Abundance correlates with favorable outcome in multiple hematological solid tumor indications
- Tumor infiltration is independent of mutational load
- Important immunosurveillance function
- Antigen-presenting function
Our Platform
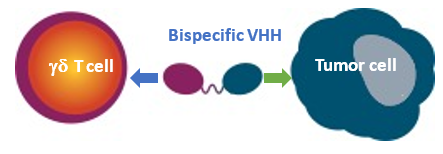
LAVA`s bispecific gamma-delta T-cell engagers (bsTCEs) directly induce potent killing of tumor cells via their unique targeting of Vγ9Vδ2 T-cells and tumor associated antigens (TAAs).
They combine a variety of traits to accomplish this including:
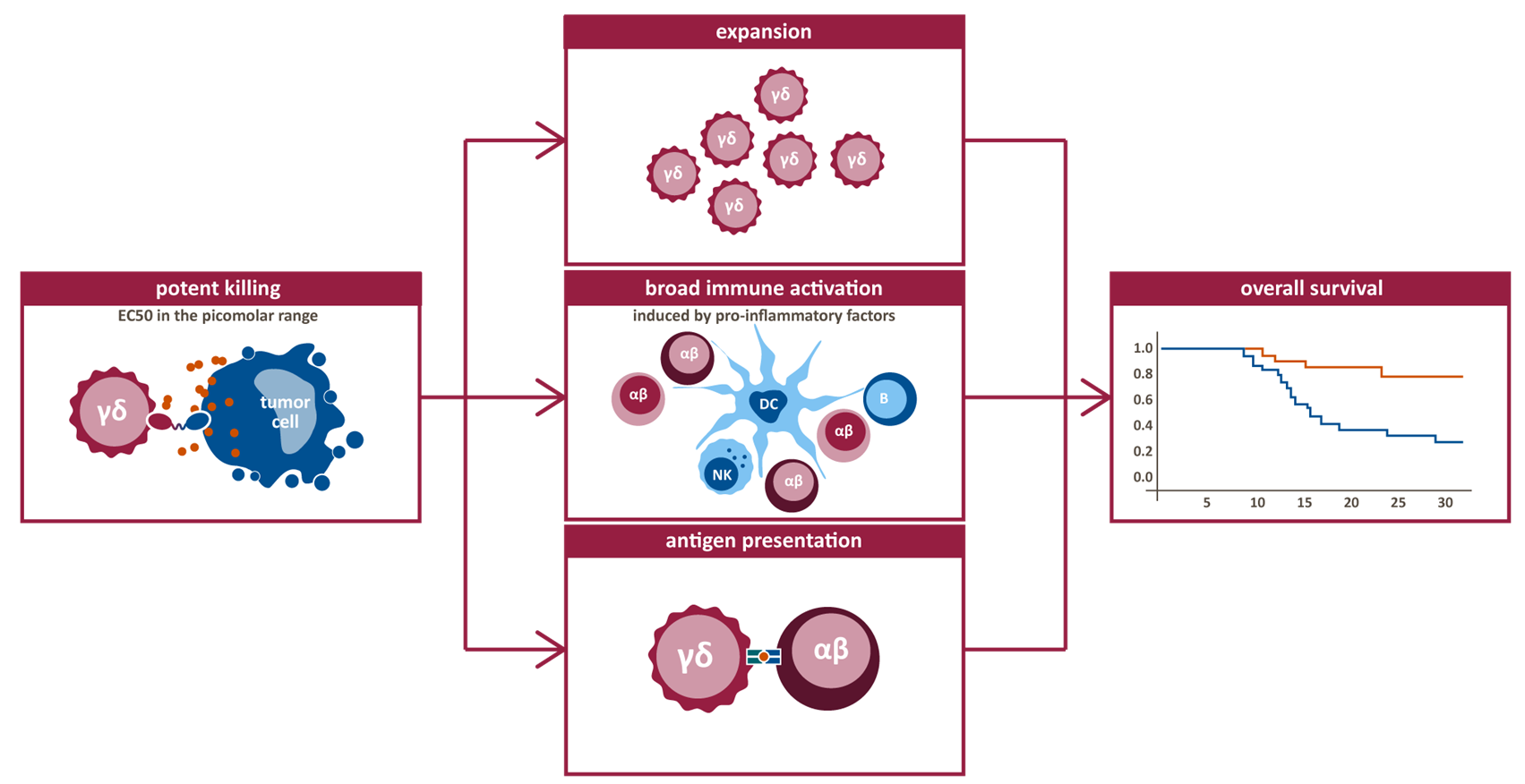
- High potency with EC50s in low picomolar range
- Potential for expansion of activated Vg9Vd2 T-cells
- No activation of immunosuppressive T-cells
- Secretion of pro-inflammatory cytokines that attract and activate other cells within immune system
- Antigen presenting capability, potentially triggering an adaptive immune reaction, enabling deep and durable responses
Proof of principle of LAVA’s approach has been established by in vitro, in vivo and ex vivo studies in which gamma-delta bsTCEs demonstrate potent cell death in both tumor cell lines and patient-derived cells in both solid tumors and hematological malignancies. Based on these data, LAVA is advancing several novel gamma-delta bsTCEs towards clinical development.
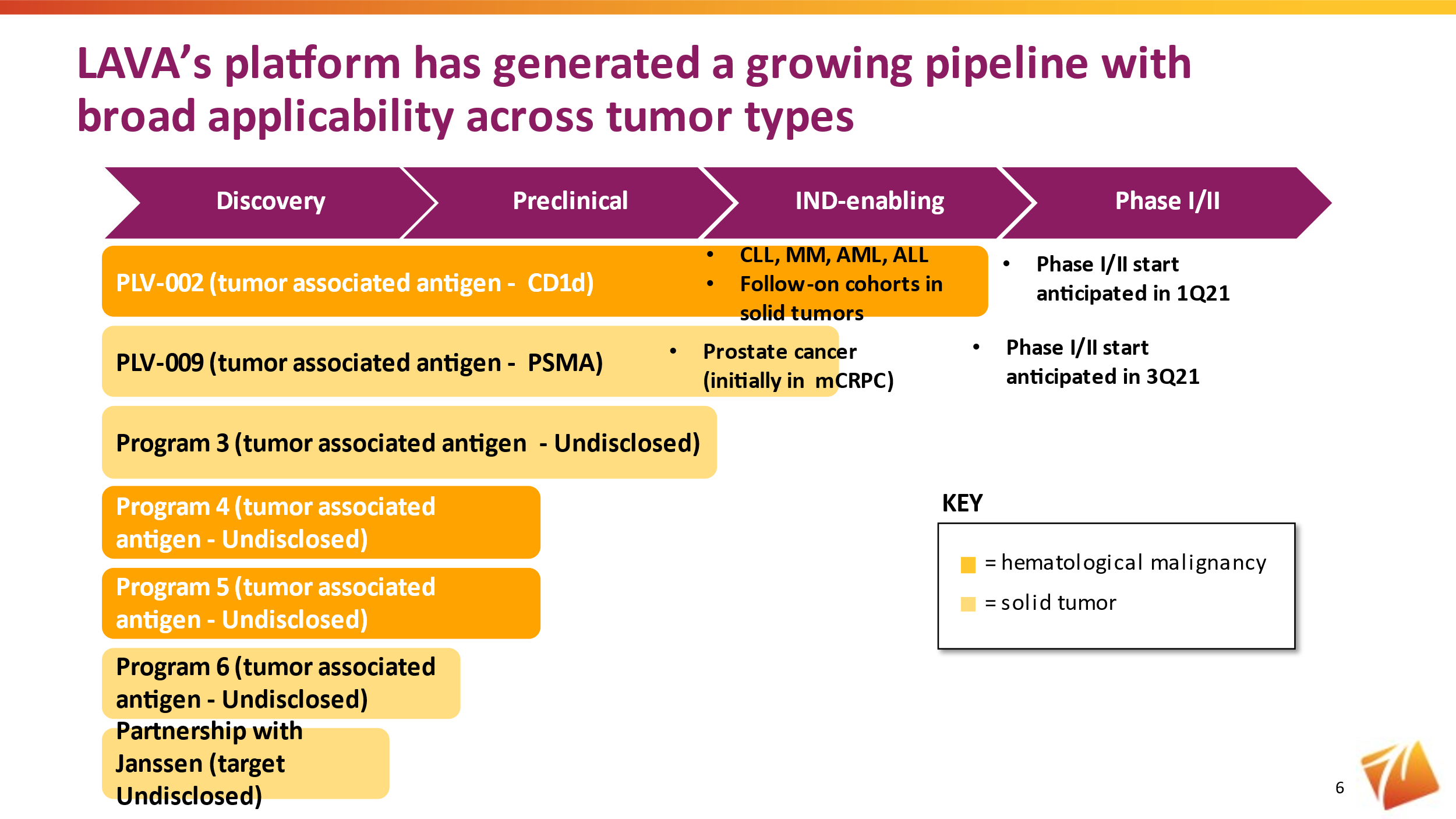
Programs
PLV-002
Our lead program, PLV-002, is a unique, humanized gamma-delta bsTCE targeting CD1d-expressing tumors, including multiple myeloma, chronic lymphocytic leukemia (CLL), and acute myeloid leukemia (AML). We have achieved preclinical proof-of-concept with PLV-002 by demonstrating efficacy and safety in a variety of preclinical models. Unique to PLV-002, it activates both Vγ9Vδ2 T cells as well as invariant natural killer T-cells, which represent another conserved immune effector cell population, in a target dependent manner.
We plan to begin a Phase I/IIa clinical trial in patients with relapsed and/or refractory multiple myeloma and CLL in the first quarter of 2021.
PLV-009
PLV-009, a gamma-delta bsTCE targeting the prostate specific membrane antigen (PSMA), has also demonstrated preclinical proof-of-concept. We expect to initiate a Phase I/II trial in metastatic castration resistant prostate cancer in the third quarter of 2021.
Early-Stage Pipeline
In addition to our two named lead programs, we are advancing a portfolio of early-stage programs, which we expect will provide the opportunity for additional INDs beginning in 2023.
Janssen Collaboration
In May 2020, we entered into a collaboration agreement with Janssen Biotech, Inc., one of the Janssen Pharmaceutical Companies of Johnson & Johnson. Under the terms of this agreement, we are responsible for discovering and developing novel gamma-delta bsTCEs specific for an undisclosed target for the treatment of cancer. We received an upfront payment, and are eligible to receive development, regulatory, and commercial milestones, as well as tiered royalties on net end-user sales.

 ABOUT US
ABOUT US